Informed Consent Is Considered An Application Of Which Belmont Principle?
Informed consent is considered an application of the Belmont principle of respect for persons. This principle emphasizes that individuals should be treated as autonomous agents, and those with diminished autonomy are entitled to protection.
Informed consent is a crucial element in research and medical ethics. It ensures that participants understand the nature of the study and consent to it voluntarily. The Belmont Report, which outlines ethical principles for research, highlights three core principles: respect for persons, beneficence, and justice.
Among these, respect for persons is directly linked to informed consent. This principle ensures that individuals are given the opportunity to make informed decisions about their participation. Understanding the connection between informed consent and the Belmont principles helps safeguard ethical standards in research practices.
Introduction To Informed Consent
Informed consent is a key concept in medical and research ethics. It ensures that individuals are fully aware of the procedures, risks, and benefits before agreeing to participate in a study or undergo a medical procedure. Informed consent protects the autonomy and rights of the participants.
Definition And Importance
Informed consent means providing clear and comprehensive information. It allows individuals to make educated decisions about their involvement. This process respects personal autonomy and promotes trust between researchers or medical professionals and participants. It ensures ethical standards in research and healthcare.
Historical Context
The concept of informed consent has evolved over time. Its roots can be traced back to the Nuremberg Code of 1947. This code was established after World War II to protect human subjects in research. The Belmont Report of 1978 further emphasized the importance of informed consent. It highlighted three core principles: respect for persons, beneficence, and justice. Informed consent is directly related to the principle of respect for persons. This principle underlines the importance of recognizing personal dignity and autonomy.
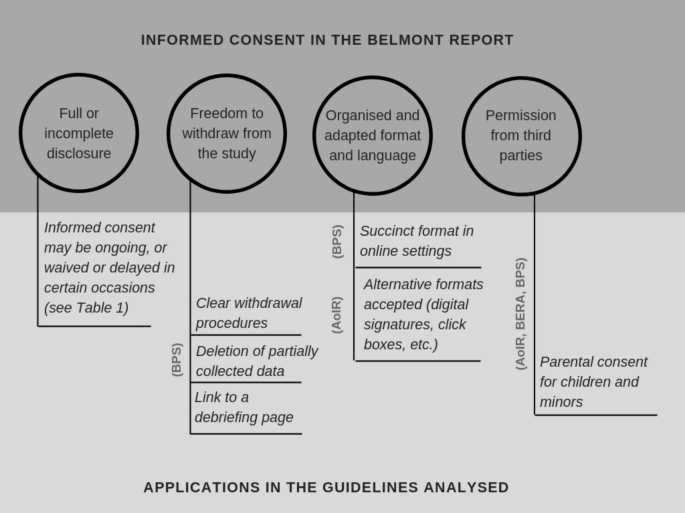
Credit: link.springer.com
The Belmont Report
The Belmont Report is a cornerstone document in the field of ethics. It was created to protect human subjects involved in research. This report lays down key principles that ensure ethical research practices.
Origins And Purpose
The Belmont Report originated in the United States in 1978. It was the result of the National Research Act. The primary purpose was to safeguard the rights of research participants. The report was named after the Belmont Conference Center where it was drafted.
The report addresses ethical issues in research involving human subjects. It was a response to past unethical studies. These studies raised concerns about the treatment of participants. The report aimed to set clear guidelines for ethical research.
Key Principles
The Belmont Report outlines three main principles:
- Respect for Persons: This principle emphasizes informed consent. Participants must be fully informed about the research. They should voluntarily participate without coercion.
- Beneficence: Researchers must ensure the well-being of participants. The research should maximize benefits and minimize harms.
- Justice: This principle ensures fairness in research. The benefits and burdens of research must be distributed equitably.
Informed consent is a direct application of the Respect for Persons principle. It ensures that participants have all necessary information. They can make an educated decision about their participation.
These principles guide ethical research practices. They protect the rights and welfare of participants. The Belmont Report remains a fundamental document in research ethics.
Principle Of Respect For Persons
The Principle of Respect for Persons is fundamental to ethical research. It ensures that individuals are treated with dignity and autonomy. This principle requires that participants are fully informed and give their consent freely. Respect for Persons is crucial in protecting the rights and well-being of individuals in research settings.
Autonomy And Protection
Respect for Persons emphasizes the autonomy of individuals. Autonomy means that people have the right to make their own decisions. In research, this involves providing complete information about the study. Participants must understand the risks and benefits before consenting. This process is known as informed consent.
Protection is another key aspect. Vulnerable populations need extra safeguards. This includes children, the elderly, and those with mental impairments. Researchers must ensure these groups are not exploited. They must take special care to protect their rights and interests.
Application In Research
Informed consent is an application of the Principle of Respect for Persons. Researchers must provide clear and comprehensive information. They must ensure participants understand the study’s purpose, procedures, and potential risks. Participants should also know that their participation is voluntary.
Researchers must create a consent form that is easy to understand. They should avoid complex medical jargon. This helps participants make an informed decision. Researchers must also be available to answer any questions participants may have.
Respect for Persons is not just about obtaining consent. It involves ongoing communication. Researchers should provide updates on the study’s progress. They should inform participants of any new risks. This continuous interaction helps maintain trust and respect.
Informed Consent And Respect For Persons
Informed consent is a cornerstone of ethical research. It ensures that individuals have the autonomy to make informed decisions about their participation. This concept aligns with the Belmont Principle of Respect for Persons, which emphasizes the importance of treating individuals as autonomous agents.
Core Components
Informed consent involves several core components that ensure clarity and understanding.
- Disclosure: Researchers must provide all relevant information about the study.
- Comprehension: Participants need to understand the information presented.
- Voluntariness: Participation must be voluntary, without any form of coercion.
Ethical Considerations
Ethical considerations in informed consent include respecting participants’ autonomy and protecting their rights.
- Autonomy: Ensuring participants can make decisions independently.
- Beneficence: Researchers must aim to do good and minimize harm.
- Justice: Fair selection and treatment of participants is essential.
Informed consent is not just a formality. It’s a commitment to ethical research. By adhering to these principles, researchers respect the dignity and rights of their participants.
Informed Consent In Practice
Informed consent is essential in research involving human subjects. It ensures participants understand the study and agree to take part. This practice upholds the Belmont Principle of Respect for Persons. Let’s explore how informed consent works in practice.
Procedures And Documentation
Researchers must follow specific procedures to get informed consent. First, they explain the study’s purpose and duration. They describe any risks and benefits involved. They must also inform participants about their right to withdraw anytime.
Documentation is crucial. Participants sign a consent form confirming they understand and agree. This form includes all the information discussed. It serves as a legal record of their consent.
Challenges And Solutions
Informed consent can present challenges. Language barriers can make understanding difficult. Researchers should use simple language and translators when needed.
Another challenge is ensuring participants truly understand the information. Researchers should ask questions to check understanding. Visual aids and interactive methods can help too.
Some participants may feel pressured to join. It is vital to assure them that participation is voluntary. They should feel no obligation or fear of repercussions.

Credit: www.managementnote.com
Case Studies
Informed consent is a crucial element in ethical research. It falls under the Belmont Report’s principle of respect for persons. Understanding informed consent through case studies helps illustrate its importance. These examples show how informed consent has evolved over time.
Historical Examples
One significant example is the Tuskegee Syphilis Study. Conducted from 1932 to 1972, it involved African American men who were not informed about the true nature of the study. They were misled and not given proper treatment, even when penicillin became available. This lack of informed consent caused harm and ethical outrage. The study led to stricter regulations to protect research participants.
Another historical example is the Willowbrook Hepatitis Study. This study, conducted between 1956 and 1971, involved children with intellectual disabilities. Researchers deliberately infected children with hepatitis to study the disease. Parents were pressured to consent by being told their children would receive better care. This study highlighted the need for genuine informed consent and protection of vulnerable populations.
Modern Applications
Today, informed consent is a standard practice in research. One modern example is clinical trials for new medications. Participants receive detailed information about the study, potential risks, and benefits. They must sign a consent form before participating. This process ensures they understand what they are agreeing to.
Another modern application is in genetic research. Researchers collect DNA samples to study genetic diseases. Participants are informed about the purpose of the study, how their data will be used, and their privacy rights. This transparency builds trust and ensures ethical research practices.
Informed consent remains a cornerstone of ethical research. Case studies from history and modern times show its evolution and importance. By learning from past mistakes, researchers today can conduct studies that respect participants’ rights and dignity.
Regulations And Guidelines
Regulations and guidelines ensure the ethical conduct of research. They protect participants’ rights and welfare. Informed consent is a key aspect. It is rooted in the Belmont Principle of respect for persons. Let’s explore how this principle is applied through various standards and guidelines.
International Standards
International standards set the stage for ethical research worldwide. The Declaration of Helsinki is a prime example. It provides guidelines for physicians conducting medical research. It emphasizes informed consent and participants’ rights. Researchers must fully inform participants about the study. They must also respect their autonomy and decision-making ability.
Institutional Review Boards
Institutional Review Boards (IRBs) play a crucial role. They review research proposals to ensure ethical standards. They focus on informed consent and participants’ safety. IRBs ensure that researchers provide clear and comprehensive information. This includes the study’s purpose, procedures, risks, and benefits. Participants must understand this information before consenting.
Future Of Informed Consent
The future of informed consent is rapidly evolving. This evolution is driven by technological advancements and changing ethical standards. Understanding these changes is crucial for researchers and participants alike. Let’s explore what lies ahead.
Technological Advancements
Technological advancements are transforming informed consent. Digital platforms are replacing paper forms. This makes the consent process faster and more efficient. Participants can now give consent online. This convenience increases participation rates.
Interactive tools are also enhancing understanding. Videos and quizzes help clarify complex information. This ensures participants fully understand what they are consenting to. Technology is making informed consent more user-friendly.
Evolving Ethical Standards
Ethical standards are also evolving. There is a growing focus on participant autonomy. Researchers must ensure that consent is truly informed and voluntary. This involves clear communication and transparency.
New guidelines emphasize continuous consent. Participants should be informed about any changes during the study. This ongoing communication respects their autonomy. Ethical standards are adapting to protect participants better.
By embracing these changes, we can improve the informed consent process. This benefits both researchers and participants. The future of informed consent looks promising.

Credit: slideplayer.com
Frequently Asked Questions
What Is Informed Consent?
Informed consent is a process where participants are fully informed about a study’s risks and benefits before agreeing to partake.
Which Belmont Principle Covers Informed Consent?
Informed consent falls under the Belmont Principle of Respect for Persons, ensuring individuals’ autonomy and protection.
Why Is Informed Consent Important?
Informed consent is vital for ethical research. It ensures participants understand the study and voluntarily agree to participate.
How Is Informed Consent Obtained?
Researchers obtain informed consent by providing clear information about the study and confirming participants’ voluntary agreement to partake.
Conclusion
Understanding informed consent is crucial in ethical research. It aligns with the Belmont Principle of Respect for Persons. This principle emphasizes respecting individual autonomy. Researchers must provide clear, comprehensible information. Participants should make informed, voluntary decisions. Ethical practices build trust and integrity.
Following these guidelines ensures responsible research conduct. Embrace informed consent to honor participants’ rights and dignity. This approach fosters a respectful and ethical research environment.



